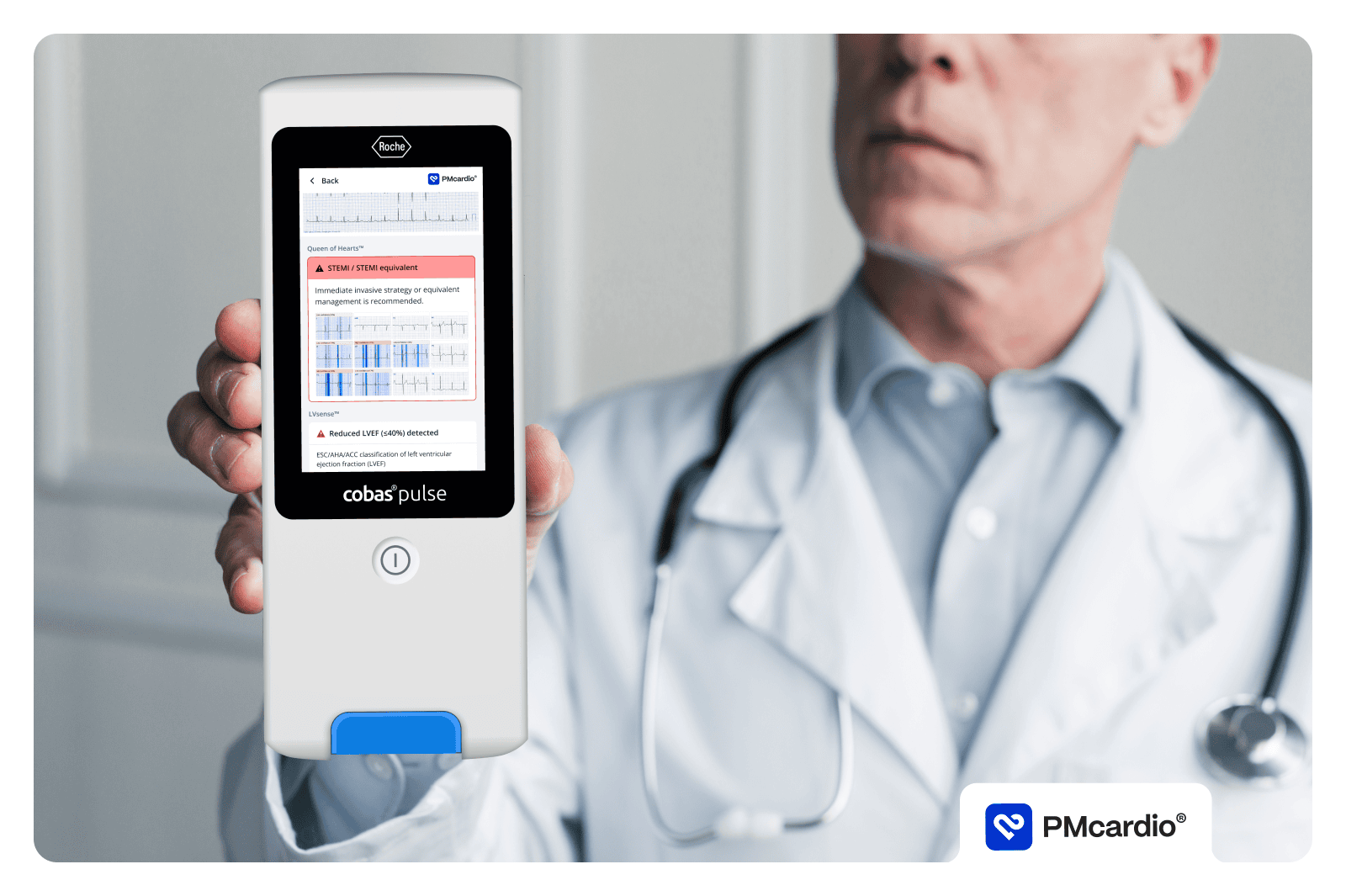
PMcardio is an AI-powered clinical assistant supporting clinicians in diagnosing and managing over 40 cardiovascular diseases. The platform is available as a user-friendly smartphone application, making it accessible to all hospital staff and primary care practitioners. The platform can be neatly incorporated into hospital and practice systems, driving clinical decisions and providing recommendations based on the latest clinical guidelines. Empowering healthcare professionals in tackling the number one cause of death globally, PMcardio can save lives and significantly reduce healthcare costs.
With the introduction of the EU MDR in summer last year in place of the EU Medical Device Directive (MDD), the medical device industry has been shaken up with stricter and neck-breaking regulations and conformity assessments – especially for medical device software.
Challenged by this change, we navigated through the complex certification process by automating most regulatory processes, concepts, and day-to-day workflows.
“We have automated many of the key regulatory processes, concepts, and day-to-day workflows, enforcing compliance across the board,” comments Simon Rovder, CTO at Powerful Medical. “At Powerful Medical, we don’t look for any solution. We don’t stop until we find the solution,” he continues.
Due to the transition from the MDD to the MDR, only a few notified bodies are still accredited to certify medical devices, which presents an additional challenge for medical device companies and startups. With all companies having to recertify due to the regulation change, the notified bodies are overwhelmed, further delaying certification times.
A recent survey from the renowned German medical device consultancy Johner Institute has demonstrated that the average time from successful audits to released certificates has increased from 1-3 months to 6-12 months, with some manufacturers waiting for over 18 months already.
Therefore, with the globally known and recognized Notified Body TÜV SÜD from Germany, we chose a strong and international partner for the entirety of Europe, including the United Kingdom which, after Brexit, requires the UKCA mark starting summer 2023.

“We’re a European company, and Europe is our key market. Having successfully obtained the CE-marking in the EU under the new regulation allows us to market the world’s first AI-powered platform for ECG Digitization, Interpretation, and Patient Management across Europe. Being certified as a class II(b) medical device allows PMcardio to not only inform decision-making but actually drive it, making it a powerful tool in the healthcare professionals’ pocket”, comments Martin Herman, CEO at Powerful Medical.
Looking at the competition, the EU Medical Device Regulation has forced many European medical device companies, especially startups, to abandon or delay their EU certification plans and pursue the US FDA approval, which, since the recent regulation change, is considerably easier to obtain than the CE-mark under the EU MDR.
“With the EU CE-mark in our pocket, we can now feel confident about the US FDA approval. Already in the process, we’re expecting the US FDA approval of our first product later this year”, comments Martin Herman.
In line with the expected FDA approval, we’re now preparing for a large Series A with US Healthcare Investors to expand our commercialization efforts beyond Europe.